Abstract
Background RM after 1L immunochemotherapy has improved the Progression-Free Survival (PFS) and Overall Survival (OS) in MCL in phase III clinical trials and in real-life patients. Early progression/relapse (E-POD) after 1L has been significantly associated with a shorter OS in younger patients with MCL after intensive 1L and autologous stem-cell transplantation (ASCT), although these patients did not receive RM (Visco et al, BJH 2019). The aim of our study was to analyze the impact of the use of RM on the risk of early-POD in a real-life cohort of patients with MCL.
Methods We retrospectively reviewed all patients diagnosed with MCL between January 2000 and December 2021 at 6 tertiary centers. Only patients receiving 1L at diagnosis and achieving complete (CR) or partial response (PR) were eligible for maintenance and therefore included in this analysis. Patients were categorized as "RM” if they had received RM after 1L or as "non-RM” if they had not. Relapse/progression after 1L occurring before or after the first 24 months (m) from diagnosis was considered early or E-POD and late or L-POD, respectively (Visco et al, BJH 2019). Patients not relapsing after 1L were considered L-POD only if their follow-up time (FU) from diagnosis was 24 months or longer.
Patient's characteristics, biological risk factors at diagnosis, as well as the type of 1L were recorded (intensive immunochemotherapy with high-dose cytarabine -HiDAC- or standard immunochemotherapy without HiDAC -non-HiDAC-), with or without ASCT consolidation. The duration of response (DOR) after 1L, PFS and OS were also analyzed for all patients.
Statistical analyses were performed with the R-4.1.0 software. Univariate logistic regression was carried out to study the association between E-POD and maintenance. Survival analysis (DOR, PFS and OS) were calculated using the Kaplan-Meier method and the univariate Cox was used for statistical comparison.
Results Out of 212 patients attaining CR or PR after 1L, 87 (41%) received maintenance therapy (RM group) whereas 125 patients (59%) did not (non-RM group). The RM regimens administered were: rituximab monotherapy (R)/8 weeks (wk) x 2 years (N=41), R/8 wk x 3 years (N=27) and other R-based regimens (with BTK inhibitors, N=7; other R schedules, N=12). Except for a higher percentage of patients older >65 years at diagnosis in the RM group (51.7% RM vs. 37.6% non-RM, p=0.042), the rest of baseline characteristics were well balanced between the two groups (Table 1). Regarding 1L, more patients in the full cohort had received a non-HiDAC regimen [120 pts (56.6%) non-HiDAC vs. 92 pts (43.4%) HiDAC], but the proportion of patients receiving RM was similar after both types of 1L. Only 22 out of the 87 patients in the RM group (25.3%) had undergone previous ASCT consolidation (Table 1).
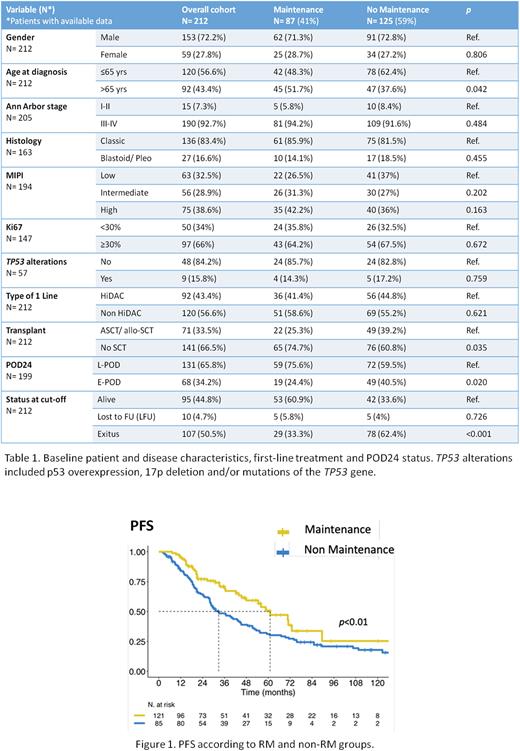
With a median follow-up of 72.8 months (51.52 m and 92.65 m in the RM and non-RM groups, respectively), median PFS was 44.39 m (36.11-55.46), with a significant advantage for the RM group (61.04 m vs. 32.82 m, HR=1.7 [1.15-2.51], p=0.01) (Figure 1). Moreover, median DOR was 30.03 m (26.02-42.94), also significantly longer in the RM group (53.88 m vs. 24.21 m, HR=1.61 [1.12-2.3], p=0.01). Median OS was 12.18 yrs (8.66-NR) in the full cohort, with no significant differences between the two groups (14.03 yrs vs. 11.48 yrs, HR=1.34 [0.74-2.45], p=0.34).
Regarding POD24, a statistically significant association was observed between not receiving RM and early-POD: 49 patients (40.5%) were E-POD in the non-RM group compared with only 19 (24.4%) in the RM group (OR=2.11 [1.14-4.04], p=0.020) (Table 1). Conclusions
Only 41% of our real-life cohort received RM despite it being considered a safe and effective strategy for MCL patients.
In line with previous studies, in our series RM after 1L is associated with a significant advantage in terms of PFS and DOR, although it did not have impact on OS.
In our cohort, RM is significantly associated with a lower risk of early-POD, in all age groups and regardless of the 1L induction strategy, therefore confirming the benefit of this strategy in the management and outcomes of MCL.
Disclosures
Cabirta:Jazz: Honoraria; Astra-Zeneca: Honoraria; Janssen: Honoraria. Abrisqueta:Roche: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Sandoz: Honoraria; Incyte: Consultancy. García:Janssen, Roche, Gilead, Celgene, Abbvie: Other: medical meetings funding; Janssen, Abbvie: Research Funding; Janssen, Roche, Gilead, Celgene: Consultancy. Sancho:Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Miltenyi Biomedicine: Consultancy, Membership on an entity's Board of Directors or advisory committees; Eli Lilly & Company: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kern Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Cordoba:Bristol Myers Squibb: Consultancy, Honoraria; Pfizer: Research Funding; Kite: Consultancy, Speakers Bureau; Takeda: Consultancy; GenMab: Consultancy; Incyte: Consultancy; Janssen: Consultancy, Honoraria, Speakers Bureau; BeiGene: Consultancy; Lilly: Consultancy; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Celgene: Honoraria; Gilead: Honoraria; AbbVie: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau. Carpio:Gilead: Honoraria; Novartis: Honoraria; AstraZeneca: Honoraria; BMS: Honoraria; Regeneron Pharmaceuticals, Inc.: Consultancy; Takeda: Consultancy. Iacoboni:NOVARTIS, KITE/GILEAD, BMS/CELGENE: Consultancy; NOVARTIS, KITE/GILEAD, BMS/CELGENE, ASTRAZENECA, ROCHE, ABBVIE, JANSSEN, MILTENYI: Honoraria. Bosch:Novartis: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Karyospharm: Honoraria; Mundipharma: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Beigene: Consultancy, Honoraria. Marin-Niebla:Lilly: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Kiowa Kirin: Consultancy; Takeda: Consultancy, Honoraria; Astra-Zeneca: Consultancy; Roche: Consultancy; Janssen: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal